A sample of blue crystalline salt X is heated in a clean dry hard glass test tube . The salt on - Brainly.in

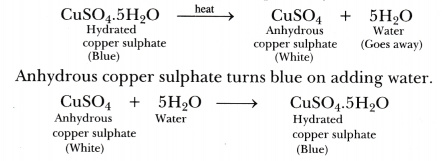
The metal salt A is in blue colour. When salt A is heated strongly over a burner then a substance B present in it is eliminated and a white powder C is

The metal salt A is blue in color. When salt A is heated strongly over a burner, then a substance B - Brainly.in

On strongly heating, a blue salt leaves a black residue. Which of the following cations can be present in the salt?

the crystallisation salt na2so4xh2o on heating losses 559 of its weight the formula of the crystalline salt is - Chemistry - TopperLearning.com | cf614k66