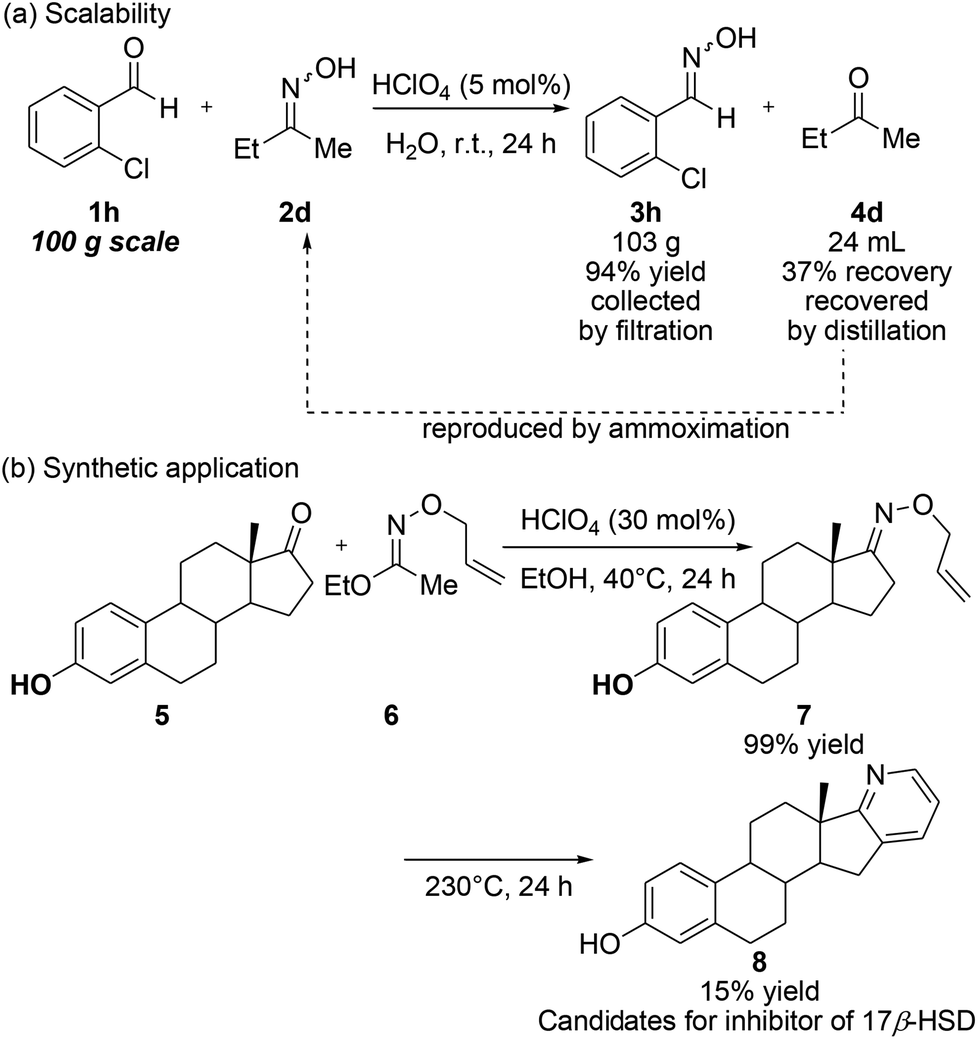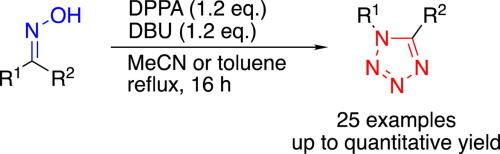
Stereospecific synthesis of 1,5-disubstituted tetrazoles from ketoximes via a Beckmann rearrangement facilitated by diphenyl phosphorazidate - Tetrahedron Lett. - X-MOL

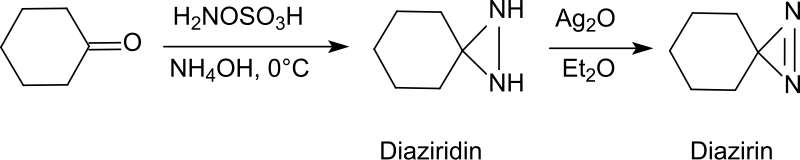
Direct and Stereospecific Synthesis of N-H and N-Alkyl Aziridines from Unactivated Olefins Using Hydroxylamine-O-Sulfonic Acids. | Semantic Scholar

Synthesis of a tricyclic lactam via Beckmann rearrangement and ring-rearrangement metathesis as key steps. - Abstract - Europe PMC
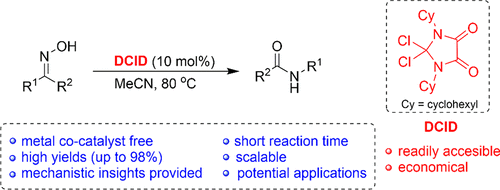
Dichloroimidazolidinedione-Activated Beckmann Rearrangement of Ketoximes for Accessing Amides and Lactams - J. Org. Chem. - X-MOL

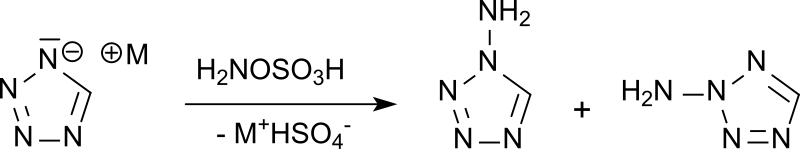
Synthesis of antiprotozoal diamines by regioselective insertion of nitrogen into a bicyclic ring system | SpringerLink

Table 1 from Cu(OTf)2-catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA). | Semantic Scholar

Cu(OTf)2-catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA). - Abstract - Europe PMC

Cu(OTf)2-catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA) | Request PDF