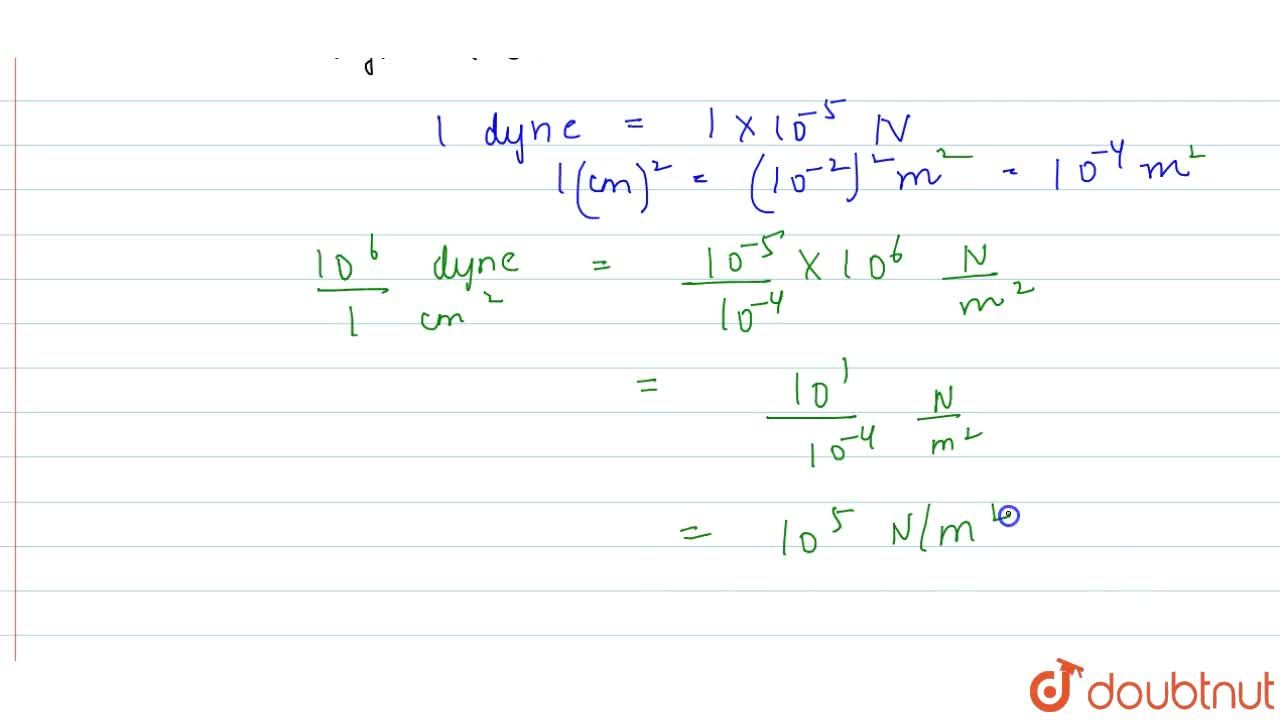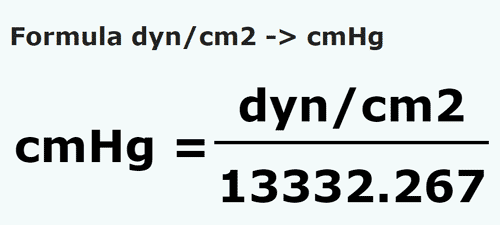SIO 210 housekeeping Tutorials: Math tutorials (finalize time) (M or W 3-4) Course material tutorials (finalize 2 times) Exam format: in-class, closed. - ppt download

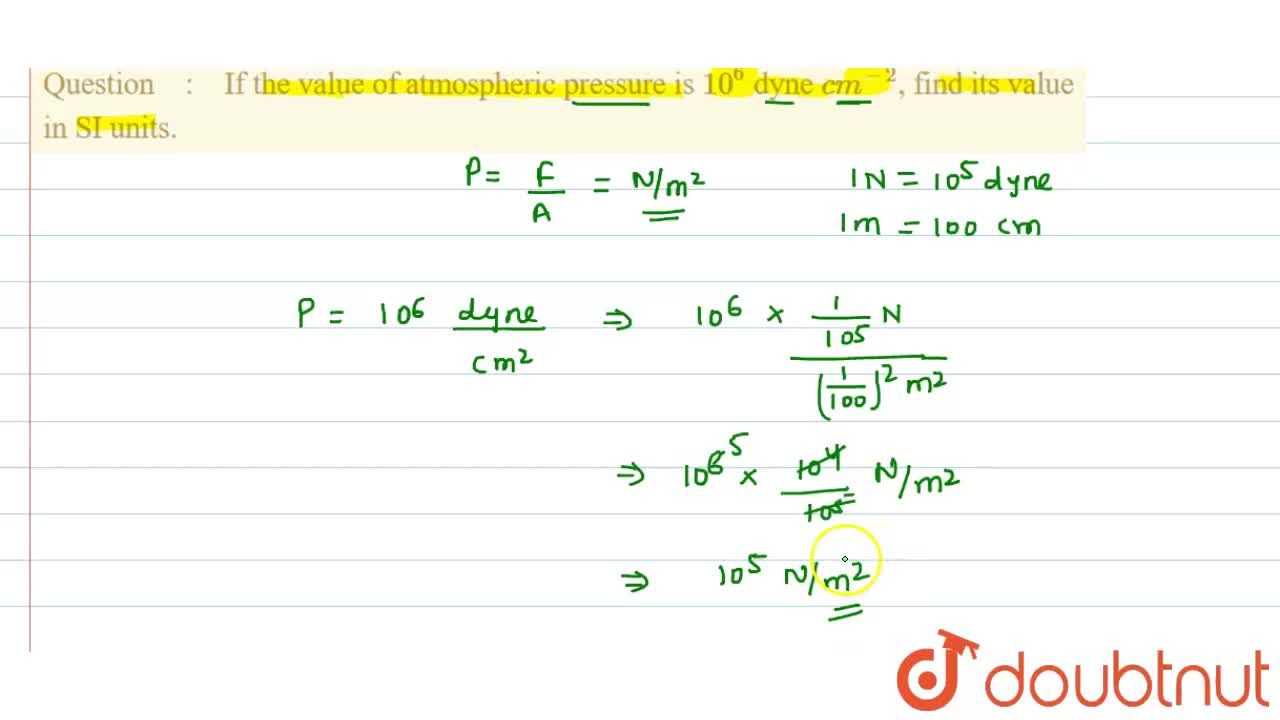
Pressure is determined as force per unit area of the surface. The SI unit of pressure, pascal is as shown below: 1 Pa = 1 N m^-2 If mass of air at
![What will be the pressure in dyne cm^-2 , due to a water column of height 10cm ? [take g = 980cm/s^2 ] (density of water = 10^3kg m^-3 ). What will be the pressure in dyne cm^-2 , due to a water column of height 10cm ? [take g = 980cm/s^2 ] (density of water = 10^3kg m^-3 ).](https://haygot.s3.amazonaws.com/questions/1966104_512604_ans_ec02bf5104b34c5eb44f2211f9ef5b50.jpg)
What will be the pressure in dyne cm^-2 , due to a water column of height 10cm ? [take g = 980cm/s^2 ] (density of water = 10^3kg m^-3 ).